Lay Summary by Daman Dhunna
Edited by Rona Herzog
This is a summary of a paper by ICORD researchers Vera-Ellen Lucci, Dr. Jessica Inskip, Maureen McGrath, Dr. Brian Kwon, Dr. Victoria Claydon, and their colleagues Dr. Ian Ruiz and Rebekah Lee. To access the original article, click here.
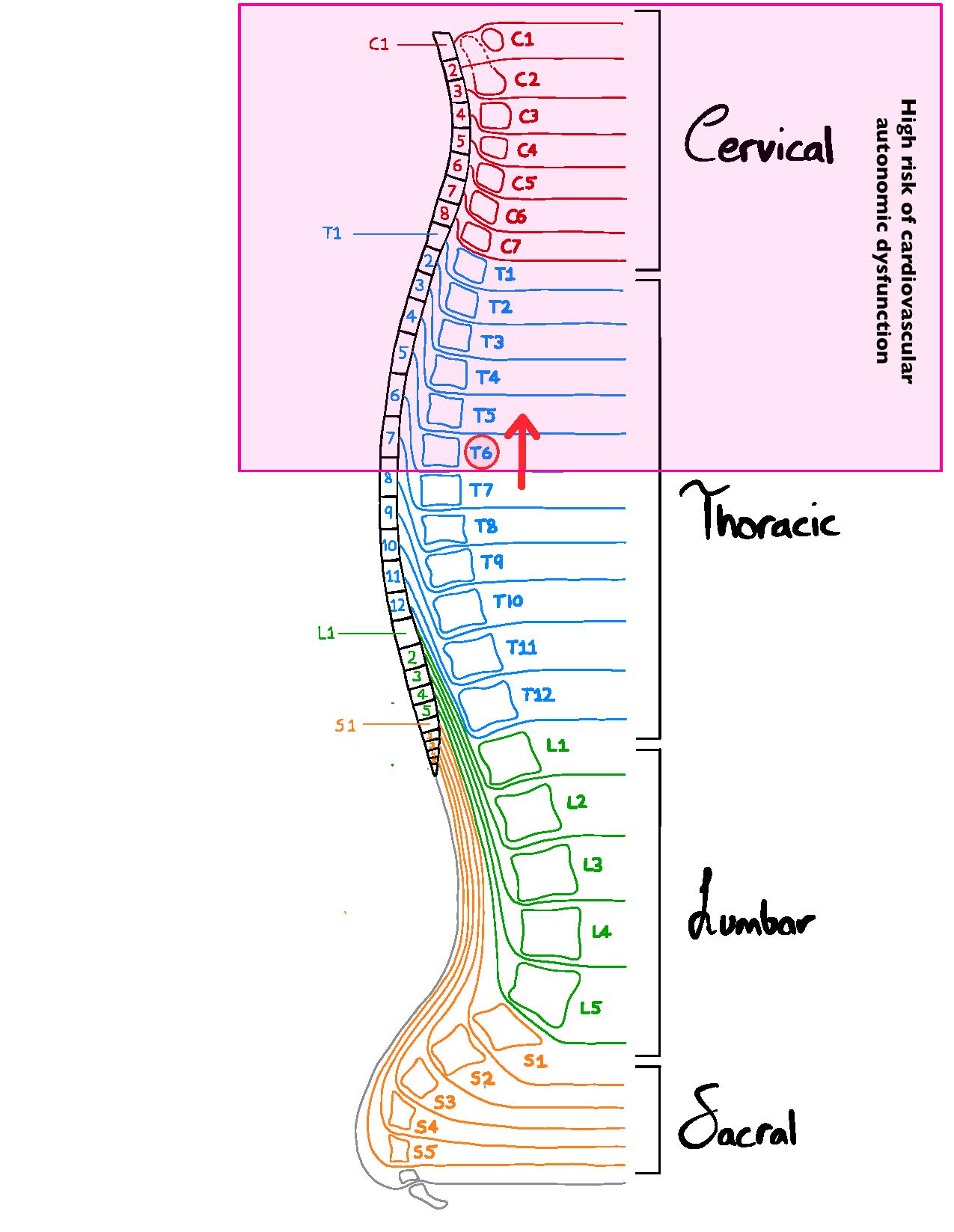 Our spinal nerves, depicted in the image on the right, innervate various organs/organ systems to modify their function. Autonomic nerves called sympathetic nerves that exit the spinal cord above T6 innervate both the heart, influencing the rate and force of the heartbeat, as well as the blood vessels, which are important in controlling blood pressure. This cardiovascular autonomic modulation of the cardiovascular system is critical for normal control of the circulation, and is important during “fight or flight” situations, known as sympathetic stimulation.
Our spinal nerves, depicted in the image on the right, innervate various organs/organ systems to modify their function. Autonomic nerves called sympathetic nerves that exit the spinal cord above T6 innervate both the heart, influencing the rate and force of the heartbeat, as well as the blood vessels, which are important in controlling blood pressure. This cardiovascular autonomic modulation of the cardiovascular system is critical for normal control of the circulation, and is important during “fight or flight” situations, known as sympathetic stimulation.
Spinal cord injuries (SCI) that affect spinal levels above T6 can severely disrupt the capacity to regulate heart rate and blood pressure, leading to cardiovascular autonomic dysfunction. This can cause problems with adjusting blood pressure and heart rate during position changes, a condition known as orthostatic hypotension (a drop in blood pressure when moving from lying to upright positions), that can lead to fatigue, and dizziness or fainting. Other problems can include an inability to increase heart rate and blood pressure during exercise, creating challenges with activities of daily living, as well as a condition of extremely high blood pressure, known as autonomic dysreflexia, that can be triggered by many different daily activities, such as a full bladder or bowel or conducting personal care routines. Individuals can also experience cardiac arrhythmias, which is defined as abnormal electrical activity in the heart.
What was the purpose of this study?
We know that individuals with SCI can experience cardiovascular autonomic dysfunction, but it isn’t known whether this occurs immediately after injury or takes time to develop, and this has important implications for attempts to prevent or treat cardiovascular autonomic dysfunction. It also isn’t clear how cardiovascular autonomic dysfunction progresses after injury, or how it relates to the cardiovascular symptoms that individuals with SCI experience. Crucially, researchers and clinicians lack simple tools to quantify the severity of cardiovascular autonomic dysfunction after SCI, making it difficult to identify and monitor those at high risk for cardiovascular autonomic dysfunction.
This study’s main goal is to:
- Track the progression of injury to cardiovascular autonomic pathways over the first year (acute phase) after injury using a novel quantitative method based on characterization of blood pressure fluctuations
- Identify individuals with severe lesions to cardiovascular autonomic pathways
- Examine the relationships between severity of injury to cardiovascular autonomic nerves and signs and symptoms of cardiovascular dysfunction
How was this study conducted?
The data in this study was collected upon 5 different visits in the following order:
- Less than 2 weeks post SCI
- 2-4 weeks post SCI
- 3 months post SCI
- 6-12 months post SCI
- More than 1 year post SCI
Each test visit comprised the following measurements of cardiovascular function, as well as self-reported cardiovascular symptom assessments:
- Lesion characteristics: level and severity of injury to movement and sensation were determined using the American Spinal Injury Association (ASIA) Impairment Scale (AIS).
- Symptom Questionnaire: filled out by participants regarding the severity of their cardiovascular symptoms (autonomic dysreflexia (AD), Orthostatic hypertension (OH), fatigue, cardiac arrythmias).
- Injury to cardiovascular autonomic pathways: with normal fine tuning of heart rate and blood pressure there are continuous fluctuations in these values with every heart beat as they are optimized for the body’s needs. When the fine tuning is lost, for example with injury to cardiovascular autonomic nerves, these values no longer fluctuate. Measures of these low frequency fluctuations in heart rate and blood pressure (low frequency systolic arterial pressure [LF-SAP] oscillations) were calculated from beat-to-beat recordings. This technique enables assessment of the extent of cardiovascular autonomic dysfunction in a simple non-invasive fashion (without poking a needle or requiring a blood sample).
- Blood pressure control: when blood pressure changes, reflex responses (the baroreflex response) would normally be activated to restore blood pressure to normal levels. From the blood pressure and heart rate readings, the baroreflex response was measured providing information about reflex control of the circulation after SCI. There are also interactions between breathing and cardiovascular control, known as cardiorespiratory interactions and these were also evaluated from the heart rate and blood pressure recordings.
- Risk for irregular heartbeats: the heart beat is controlled by electrical signals and changes in this electrical activity can be associated with increases in risk for irregular heartbeats. These electrocardiogram parameters were evaluated at each time point.
Measurements from this study were compared with previous research on chronic SCI, as well as able-bodied controls in order to gain a comparison and better understanding of the effects of injury to cardiovascular autonomic control in the acute phase of SCI.
What are the findings of this study?
Individuals with autonomically-complete lesions, meaning those with severe injury to the spinal cardiovascular autonomic pathways, could be readily identified using the new approach of measuring LF-SAP. Those with severe autonomic injuries lost fine tuning of blood pressure and so had low levels of LF SAP that did not improve over time. These analyses were best performed at 1-month after injury and did not reliably detect autonomic impairment before this timepoint. Cardiovascular symptoms were common and worsened with time, particularly in those with severe injuries to cardiovascular autonomic nerves. Baroreflex and cardiorespiratory regulation were also impaired after SCI, and there was an increased risk for irregular heart beats that was present immediately after injury and did not improve over the first year.
Overall, the researchers in this study were able to quantitatively measure the extent and impact of autonomic dysfunction in individuals with SCI by performing a simple continuous blood pressure measurement. This is clinically advantageous in many ways as it allows doctors, researchers and clinicians to better classify the presence, symptoms and signs of autonomic dysfunction after SCI without the need for arduous autonomic function tests or relying simply on symptom reports.
Why are the findings important for the SCI community?
This study provides the research and clinical communities with a distinct tool for assessing cardiovascular autonomic function after SCI, providing quantitative information about injury classification and cardiovascular consequences of injury that are not currently included in the classification of SCI. This information can change the way we understand and treat the cardiovascular autonomic complications of SCI, and fills a gap in the clinical evaluation of SCI. Assessing and measuring cardiovascular autonomic dysfunction will facilitate new research into treating the challenging cardiovascular complications of SCI. This, in turn, can be used to improve quality life for individuals living with SCI.

