Sepehr Kamal, with images created by Vivian Yeung
Edited by Ana-Maria Oproescu
This is a summary of a review article published by ICORD researcher Dr. Matt Ramer and his colleagues. In this paper, the authors reviewed 170 studies of neuropathic pain in animals following spinal cord injury (SCI) and identified several trends. They focused on why animal models are needed in addition to clinical studies, and how current animal models can be improved to better model human SCI.
Click here to access the original review article, published in The Journal of Neuroscience Research.
What is neuropathic pain?
Pain typically occurs when damage to the body creates a signal that is relayed to the brain by the nervous system. Neuropathic pain, on the other hand, is pain resulting from damage directly to the nervous system. Some of the sensations neuropathic pain can cause include burning, shooting, and pins and needles.
The pain can be continuous or it can vary from time to time. It can occur in response to a normally non-painful stimulus, or even arise with no stimulus at all. Neuropathic pain is common in conditions such as SCI, diabetes, and some vitamin deficiencies, and it can significantly impair quality of life. It is also very difficult to treat, which makes it important to develop new methods to treat neuropathic pain in affected individuals.
Why are animal models needed to study neuropathic pain following SCI?
According to the authors, lack of understanding of why neuropathic pain occurs following SCI is a major barrier to the development of new treatments for patients. They explain why rigorous scientific studies are required to improve our understanding. While clinical trials with human patients are essential, these have limitations because of important ethical constraints. For example, it would be completely unethical to induce SCI in healthy individuals. Studies with animals on the other hand allow researchers to perform intricate manipulations and make detailed observation of the outcomes in a controlled environment that would otherwise be impossible with human patients. They explain these animal studies are essential to understand how and why SCI causes neuropathic pain.
What are the problems with current animal models?
The authors argue that a disadvantage of using animal models as opposed to human patients is that an animal cannot communicate their perceived pain in the same way a human can. This makes measuring pain in animal models a challenge. Methods such as measuring an animal’s withdrawal from a painful stimulus do not consider the emotional component of pain. It is well known that emotions influence the level of perceived pain in humans, so this is an important consideration. These shortfalls limit the ability to determine whether a treatment designed to reduce pain in an animal has been successful.
Another shortfall they describe is in regard to spontaneous pain. Spontaneous pains are burning and tingling that arise without any stimulus at all. It is very common in SCI; however almost no animal studies have been conducted on spontaneous pain. This is particularly concerning as spontaneous pain typically impacts the quality of life in SCI patients more than any other type of pain.
Also, in over 90% of the studies examined, the SCI in the animal model was at the thoracic level. This was the most practical location for researchers and it was also less traumatic for the animals. However, they explain this does not represent human SCI very well as most human SCI occur at the cervical level. In fact, it is actually patients with cervical SCI that are the most likely to experience neuropathic pain, which makes it even more important to develop accurate animal models of human cervical SCI.
What insight has been gained from these studies?
Despite these shortcomings, the authors describe how animal models have provided several significant insights into the mechanisms of neuropathic pain in SCI. These studies have focused not only on spinal cord neurons, but also on neurons found in peripheral nerves and in the brain.
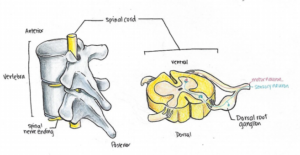
Neuropathic pain may result from an increase in the activity of the “dorsal root ganglion”, illustrated here.
For example, they mention how certain neurons found just outside the spinal cord, known as the dorsal root ganglion, show increased activity at levels below the SCI. Using animal models, researchers have been able to attribute the increased activity of dorsal root ganglions to increases in specific proteins inside these cells. Dorsal root ganglion neurons are responsible for transmitting sensory signals, such as the sensation of pain, to the brain where it is interpreted. Therefore, the increase in the activity of dorsal root ganglion neurons due to the SCI could be misinterpreted as pain by the brain.
What changes can be made to improve animal models?
The authors recommend future studies should aim to model cervical SCI injuries and to study spontaneous pain, as these are the most common types of injury and the most troublesome type of pain in human SCI. To study spontaneous pain, they suggest new methods need to be developed for measuring pain in animals. This could involve using non-invasive imaging techniques such as fMRI to detect “pain patterns” in the brain. fMRI is a technique used to measure patterns of brain activity. By comparing brain scans of humans who are experiencing pain to those who are not, researchers can determine the brain activity patterns that are unique to individuals experiencing pain. Researchers could then look for these same brain activity patterns in animals to infer the amount of pain that is being perceived by the animal.

